Clinical Laserthermia Systems – This was a cause before it was a company - From Research to Commercialization through Innovation
2018-10-19 11:02, Edited at: 2018-11-13 20:57Please note: Community posts are written by its members and not by Redeye’s research department. As a reader you’re always encouraged to critically analyze the content.
The company’s unique method imILT will as of November 2018 reach another milestone when clinical follow-up results are reported to the EU Horizon2020, a method which the company estimates will achieve $ 1 billion a year in sales revenue. CLS move towards new market areas led it to announce that it aims for market shares that will bring about $ 350 million a year in the FLA and Image-Guided Laser Ablation segments, but now, thanks to a recently announced global licensing and distribution agreement with MRI Interventions Inc. it’s time to reevaluate.

Introduction
During his twenty years of research Professor Karl-Göran Tranberg came to an understanding of how to stimulate the immune system into attacking cancer cells on a systemic level. His method of choice was to gently apply thermal heat to a solid tumor by inserting a laser fiber inside it. The precision and steadiness of the laser created the ability to withhold a temperature of approximately 46 C degrees at the tumor border for circa 30 minutes without damaging healthy surrounding tissue. His method allowed intact tumor antigens to be released and stimulate a tumor specific systemic immune response. At a glance, perhaps it seems like a simple treatment principle but the imILT procedure requires high precision instruments and effective guidance control. When the immune system is triggered by the imILT treatment not only the treated tumor dies but also distant metastases, this abscopal effect is the result of a systemic immune reaction causing the natural born killer cells, T-cells, to seek out and destroy cancerous cells in the body. This immunological process also, in principle, sets a vaccine-like immunological memory which ensures the treated cancer doesn´t return. Tranberg’s early pre-clinical and clinical research (72 patients) has shown great promise and last year CLS presented a poster at the Society of Immunotherapy of Cancer which was titled, “Anti-tumor effects and immunological response following immune stimulating interstitial laser thermotherapy” and described the initial experiences from CLS pre-clinical trials and laid out evidence for the abscopal effect and immunological memory generated by the imILT treatment when tested in mice.
Tranberg's research in the field has laid the foundation to the world’s first medical device-based immunotherapy. The method primarily addresses the large number of patients who fail to respond to CI immunotherapies (Checkpoint Inhibitors). This group represents approximately 60-65 percent of the total number of addressable patients. The research concerning CI´s recently came in to spotlight when two immunologists, James Allison of the United States and Tasuku Honjo of Japan, won the 2018 Nobel Prize in Medicine. The Nobel award recognizes their research on two different checkpoint inhibitor molecules - the “brakes” of our immune system - that when turned off, boost the body’s immune response against cancer cells faster and more effectively.
“Given the different approaches, it is likely that a combination of these methods, imILT and treatment with checkpoint inhibitors, would reinforce the effect of the individual method for many patients. Allison and Honjo's research are groundbreaking and the fact that these two receive the Nobel Prize in medicine is very gratifying” /Professor Karl-Göran Tranberg, co-founder and board member of CLS.
Since checkpoint inhibitors has no or lesser effect on, for example, breast cancer, pancreatic cancer, prostate cancer and most types of colon cancer the need for combinatorial treatment is as great as ever. Prof. Tranberg suppose the combination of CI´s and imILT holds great promise to unleash even higher efficiency rates and thereby help even more patients fight cancer. In theory the combination of imILT and immunostimulatory drugs would allow for reduced drug doses which in turn lowers the cost and risk of drug-related side effects. The total addressable market for imILT is estimated annually to amount to approximately 6 million patients globally and 560,000 patients in the two initial markets, United States and Europe.
Short-term CLS aims to generate revenues primarily through the sale of single-use products used during each treatment procedure. CLS to date carry a wide range portfolio of single-use products ranging from different Laser applicators performing a range of ablation sizes and fiber lengths to MR introducers and temperature probes. The company also offers its own laser system called the TRANBERG®|Thermal Therapy System. The system has been developed for image guided high precision thermal therapy procedures within interventional oncology and is approved for soft tissue ablation (CE and 510k clearance). The system includes a computer controlled mobile laser unit with touch screen graphical user interface. Laser light and optical fiber applicators has been specifically developed for MR-guidance and MR-thermometry, all in which are designed to greatly reduce image and feedback disturbance to maintain the highest efficiency when precision matters. For CT/US-guided procedures the TRANBERG®|Thermal Therapy System is equipped with an integrated comprehensive tissue temperature feedback system for interventions when MRI availability is scarce.
Long-term, revenues will also come from sales or lease of the TRANBERG® | Mobile Laser Units and technical service. The price for the single-use products is coupled to the health economic value of the treatment which is significant, and the single-use products are therefore positioned as premium products, offering substantial benefit to healthcare providers in the treatment of tumors. CLS target customers are hospitals and specialist clinics involved in treatment of cancer. Targeted user groups include medical specialists in surgical oncology and oncologic interventional radiology. The first TRANBERG® | Mobile Laser Units has been bought by a US hospital and several patients, with early stage prostate tumor disease, have been treated using CLS single-use products at well-renowned clinics like UTMB, Desert Medical Imaging and Toronto General Hospital. With the unique diffusing fiber technology patented by CLS, heat distribution in tissue is optimized and the need for external cooling has been made obsolete, features which significantly benefits workflow and reduce procedure time while enhancing treatment outcome.
Progress on several levels
On March 8, 2018, CLS announced that within the scope of its ongoing clinical studies regarding imILT® treatment of pancreatic cancer, a positive trend with an average time of survival of twelve months could be observed for the treated patients data stands well compared to published results for locally advanced, unresectable pancreatic cancer, which shows a median time of survival significantly under one year – in some reports nine months. No serious side-effects were noted, which is extremely positive considered that the pancreas is located close to very sensitive structures. In fact, the pancreas is an organ of the digestive system located deep in the upper part of the abdomen, behind the stomach and in front of the spine. So, it´s fair to say, once again, that precision matters.
And as of July 31st, this year, CLS closed the patient-enrollment in the three clinical studies of imILT within the EU Horizon2020 study program. The company will now submit follow-up documentation to the H2020 as of November this year. The event will upon completion add a cash contribution of approximately SEK 4 million to CLS in accordance to the EUR 2.1 million SME grant which the company won back in 2016. The three clinical studies included imILT treatment for patients suffering from Pancreatic cancer in two of the trials which were sited in Institut J. Paoli et L. Calmettes (IPC), Marseille, France and Portuguese Oncology Institute of Porto. And the third trial addressed imILT treatment of Breast cancer, this trial was performed at Nottingham Breast Cancer Institute, United Kingdom. At the point of closing the enrollment the Marseille trial had recruited five out of five patients with stage III inoperable pancreatic cancer. Most of the patients in the Porto based trial were recruited while two out of five patients were recruited in the Nottingham trial. Pancreatic cancer takes 91 percent of its victims within five years and the average survival time from point of diagnosis is well below 1 year. Pancreatic cancer has no early warning signs, and there are currently no effective screening tests. As a result, pancreatic cancer is usually discovered late. Often, the diagnosis is not made until the cancer has spread to other areas of the body (stage IV). However, research focused on better diagnostic tests and newer treatments provides a more optimistic future for patients diagnosed with pancreatic cancer. In fact, a blood test and better scans are in development.
“Before 2030, pancreatic cancer is expected to be the second-leading cause of cancer-related deaths in the United States, second only to lung cancer. In 2018, the American Cancer Society estimates there will be 55,440 new cases of pancreatic cancer, 44,330 of which will die.”
The current treatment rationale of managing pancreatic cancer is to first cut it away and put the patient on a systemic chemotherapy and when curative surgery isn´t possible the available options are palliative surgery (symptom relief), systemic chemotherapy, radiation therapy and immunotherapy. Regarding biological therapies like immunotherapy the only to date approved drug is Keytruda (Merck) for pancreatic cancer. This treatment has proven effective for patients with mismatch repair deficient tumors and this specific deficiency is found in 1 in 50 advanced pancreatic cancers. Holding these facts in mind and contemplating the lack of effective treatments, the potential of an immunotherapy like imILT holds a disruptive aspect which widens the thought to not only maintain the disease in a chronic state with sustained quality of life but also curing pancreatic cancer either as a mono- or combinatorial therapy.
“During and after imILT® intact, non-coagulated tumor antigens are released and exposed to the immune system. The treatment enables a beneficial tumor immune microenvironment. Antigen presenting cells (APCs), such as dendritic cells (DCs) and macrophages, present the tumor antigens to T cells, which are activated and becomes cytotoxic. The result is systemic immune activation against remaining tumor.”
imILT – Pancreatic cancer, Marseille, France ClinicalTrials.gov Identifier: NCT02973217
“The purpose of this trial is to evaluate efficiency when it comes to local tumor destruction of the imILT treatment method in patients diagnosed with pancreatic cancer. The purpose is also to investigate the functionality and safety as well as understanding of the subsequent immunological effects.
This trial is prospective, open and non-randomized. Five patients diagnosed with pancreatic cancer will be treated in this trial, which is estimated to be carried out during a time period of 36 months.”
imILT – Pancreatic cancer, Porto, Portugal _ClinicalTrials.gov Identifier: NCT03187587
“The purpose of this trial is to evaluate efficiency when it comes to local tumor destruction of the imILT treatment method performed percutaneously in patients diagnosed with pancreatic cancer. The purpose is also to investigate the functionality and safety of the method.
This trial is an open-label, double-arm study. Twenty patients diagnosed with pancreatic cancer will be treated in this trial, ten receiving imILT treatment and ten receiving standard chemotherapy. The study is estimated to be carried out during a time period of 21 months.”
imILT – Breast cancer, Nottingham, United Kingdom ClinicalTrials.gov Identifier: NCT03039127
“The purpose of this trial is to investigate the functionality and safety of the imILT treatment method in patients diagnosed with breast cancer. The treatment method has successfully been used for treatment of patients with breast cancer and malignant melanoma. Treatment of breast cancer patients caused an increase of cytotoxic T lymphocytes in the treated tumor, as well as activated dendritic cells at the tumor border. Regulatory T lymphocytes decreased in the regional lymph nodes.
This trial is explorative, prospective, open and non-randomized. Five breast cancer patients will be treated in this trial, which is estimated to be carried out during a time period of 9 months.”
Even though the information that is public right now is limited it simultaneously provides well needed hope for patients fighting pancreatic cancer as well as strengthen CLS medicinal claims regarding imILT. Looking past these studies, the company's intention is to continue discussions with the participating hospitals; Paoli-Calmettes Institute, Portoguese Institute of and Nottingham University Hospital. Further collaborations with these hospitals may involve a larger multi-institutional clinical study aiming to fully exploit the immunological effect of imILT. But, it may also mean that the company may begin to generate revenue from imILT® treatments, and it´s not unlikely to presume that CLS will receive its first imILT revenues during the year to come.
Through partnership towards stronger market offerings
CLS pursuit of a complete product offering has led the company to sign a Memorandum of Understanding (MoU) with Exact Imaging, Toronto, Canada with the aim to enter into a commercial collaboration on Image Guided Focused Laser Ablation (FLA) of disease in prostate. Exact Imaging is a proclaimed world leader in high resolution Micro-ultrasound systems enabling real-time imaging and biopsy guidance for the prostate. The ExactVu system operates at 29mhz and Micro-ultrasound resolution is comparable to MRI and measures a 300% improvement over conventional ultrasound. The partnership shall bring about a new disruptive product, jointly optimized for high resolution micro-ultrasound guided focused laser ablation (FLA), the goal is to offer the urologic market a superior solution for image guided focal therapy in patients with localized prostate cancer and BPH.
The forge between the Tranberg and ExactVu systems is ongoing and to accompany the hardware forge CLS has taken steps to supplement the hardware with superior software. And that´s where French based Imaged Guided Therapy (IGT) comes in to play. The two companies signed a term sheet on June 27th this year stating the main conditions of the cooperative agreement. Just two days ago, in line with the stated previous terms, the deal has now been formalized into a final agreement were the two companies plan to launch its joint product during the second half of 2019. The actual purpose of the collaboration between CLS and IGT is to jointly develop and commercialize a software that, with the aid of magnetron (MR), provides the physician with optimal conditions for measuring and controlling temperatures during treatment with focused laser ablation or CLS imILT® method. Through the collaboration with IGT, a new generation of thermometry software will be commercialized and not only contribute to CLS cash flow but also significantly improve real-time monitoring of heat distribution in the ablation zone in an MRI setup and there is reason to believe that the new software will also work well with micro-ultrasound provided by the ExactVu system, but it´s not yet confirmed. The two partners expect to have the new software approved and ready for launch in the second half of 2019.
On Oct 17th, CLS announced a new partnership, together with MRI Intervention Inc. they will enter into a collaborative license and co-development agreement to provide next-generation navigation and laser ablation platforms for use in Spine and Neurosurgery. This is a major move for CLS, to enter the neurosurgery market which is their premier contenders playground. Medtronic owned Visualase has positioned itself as the leader in the laser ablation field of neurosurgery and Monteris Medical has been a clear runner up but has had its share of troubles with FDA recalls, which to be fair, Visualase has as well.
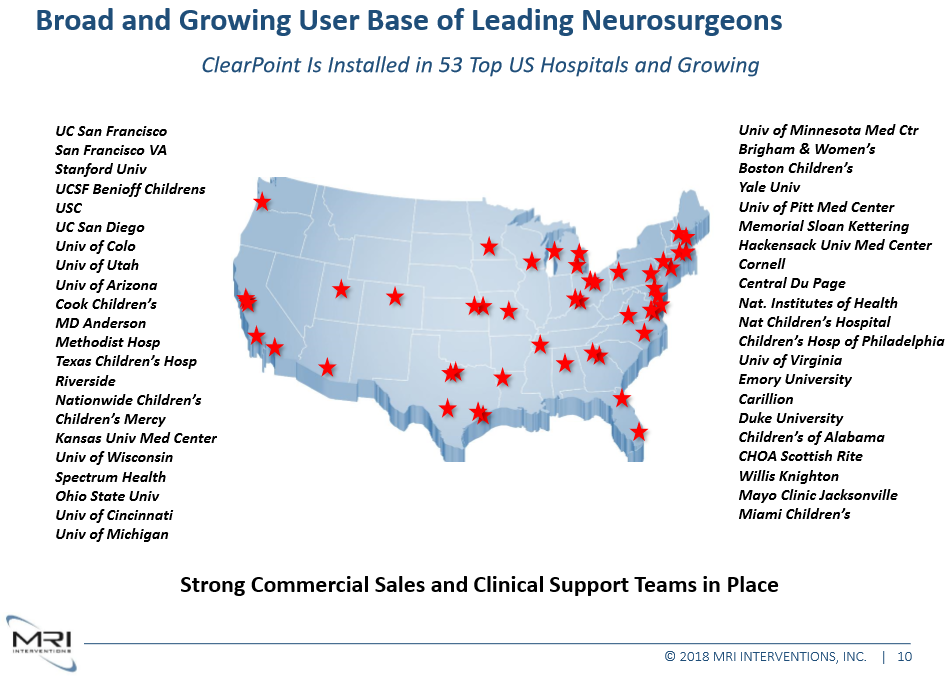
This distribution deal will allow CLS to gain access to a team of more than 20 sales managers and clinical specialists. In addition, the Swedish innovator will be able to benefit from MRI Interventions established relationships and network of hospitals and clinicians. MRI Intervention Inc. has installed its Clearpoint system in 53 top US Hospitals and are continuously growing. The two-year distribution agreement includes all interventional MR-guided procedures offered today by CLS, beyond the global licensing agreement concerning spine and neurosurgery, MRI Interventions will receive full rights to distribute and sell CLS´s portfolio of products in the US and Canada. The impact of this deal is yet to be seen but it sure adds to CLS potential and forward-looking value.
We are excited about the immediate potential in this partnership and look forward to teaming up with this great company,” stated Dan Mogren, Chief Commercial Officer of CLS
The global MRI-guided Neurosurgical Ablation market is expected to be worth around US$ 216 million in 2028 alone and its projected to increase at a CAGR of 4,9%. North America is the largest market to date, which accounted for US$ 62,5 million in 2017, and this market alone is expected to grow at CAGR of 4,3%. Some of the key players operating this field is as mentioned Medtronic along with Boston Scientific Corp. Insightec Lmt, AngioDynamics Inc, Monteris Medical Inc and CLS partner, MRI Intervention Inc. Innovation and partnership are two major drivers followed by advancement in manufacturing of MRI guided neurosurgical ablation devices and disposables in this market and leading manufacturers are focusing on collaboration to promote the use of MRI-Guided Neurosurgical Ablation for the treatment of brain tumor. Some companies are focusing to collaborate directly with hospitals to promote their MRI-Guided Neurosurgical Ablation system directly. Noteworthy is that few players in this market focus on collaboration with distributors to expand their regional presence which is why the partnership between CLS and MRI Intervention Inc. is a bit extraordinary.
“This agreement is a truly exciting step towards execution of our four-pillar growth strategy,” commented Joe Burnett, President and CEO of MRI Interventions. “Historically we have been the company that helps deliver other company’s therapeutic products to deep structures of the brain in the most precise manner possible. Now we have the opportunity to deliver our own therapies, which will improve efficiency, workflow and procedure times for our hospital customers, and expand revenue and per-procedure profitability for our investors. Now in partnership with CLS, we can truly be a leader in the movement toward minimally-invasive neurosurgery because we can offer both navigation and laser ablation therapy, all under the watchful eye of a single clinical specialist to help support the procedure.”
“We will innovate and develop new products and workflows in collaboration with CLS until we have a platform that provides accuracy and workflow advantages to our customers and meets our requirements of being able to do multiple procedures in the same day. We estimate a formal launch of this combined platform sometime in 2020. Given the advanced development and regulatory status of the Tranberg system, we see the development costs in 2019 as more of a re-prioritization of existing resources than a large operating investment. Moreover, we expect this partnership to improve profitability in 2020 given the added therapy revenue MRI will see on the top line with minimal added distribution costs through our existing sales channel and support network.”
Robotics and AI - Machine Learning
“Data-driven artificial intelligence (AI) could help contribute to better prostate cancer diagnosis and treatment, according to researchers at Sweden’s Science for Life Laboratory”
As we enter the era of digital pathology CLS has taken its first steps towards broadening its offering through commercial collaborations and I except more is to come. The bottom line, CLS is moving forward and continues to innovate while enhancing its market offer to attract the costumer’s attention even faster.
“The need for effective management of prostate cancer is as pressing as ever”
Intense development takes place in several directions and with the help of AI researchers now look to minimize the human factor in, among other things, diagnostics but also regarding the control and calculation of medical interventions. A clear link between CLS and this trend is once again the partnership with Exact Imaging. The Canadian company recently announced a plan with Cambridge Consultants where it intends to utilize years of medical technology expertise and the most important in machine learning. Cambridge Consultants is applying deep learning to high-resolution micro-ultrasound imaging to identify potential suspicious regions of tissue and inform urologists who may want to consider this additional data in their biopsy protocol. Early results show real promise.
In recent years Cambridge Consultants has been at the forefront of advances in machine learning and deep learning, applying this transformative technology to a wide range of industries and disciplines. The AI tools provided by Cambridge Consultants should be able to interpret the full amount of data obtained through the feedback from the micro ultrasound when correlated with pathology. This in turn will provide higher accuracy and better characterization of suspect areas.
Robotic prostate surgery using the Da Vinci system has helped surgeons reach superior results and minimize risk compared to traditional surgery, but the risks remain, and surgery means the patient faces the risk of incontinence and erectile dysfunction among other side effects. In 2018, the American Cancer Society (ACS) estimates that 164,690 new cases of prostate cancer will be diagnosed and 29,430 will die due to the prostate cancer in the United States (Siegel et al., CA Cancer J Clin 67:7–30, 2018). Many men with prostate cancer are often managed with aggressive therapy including radiotherapy or surgery. No matter how expertly done, these therapies carry significant risk and morbidity to the patient’s health related quality of life with impact on sexual, urinary and bowel function (Potosky et al., J Natl Cancer Inst 96:1358–1367, 2004)
Minimally invasive interventions like Focal Laser Ablation (FLA) carry superior efficiency and at the same time it almost eliminates the risk of life changing side effects. Logically when robotics is applied to FLA procedures it eliminates human error regarding guidance much like stereotaxis has done to date for biopsy guidance. One could suppose applying deep learning to this area as well would enable even more accurate treatment plans along with well calculated ablation estimates. The promise of AI and deep learning in the field of medical technology and cancer interventions allows for the thought of close to fully automated interventions with greatly reduced pressure regarding workflow and hospital resources in a nearby future.
FLA & The Key Opinion Leaders working with CLS
Dr. Eric Walser, Chairman of Radiology, UTMB at Galveston, Texas. Board Certified, Diagnostic and Interventional Radiologist. Dr. Walser once practiced medicine at the world-renowned Mayo Clinic’s campus in Florida where he was researching focal laser ablation (or surgical removal) of cancer from the lungs and liver. While researching these methods of treatment, Dr. Walser saw the opportunity to make a difference with those suffering from prostate cancer. Dr Walser has been performing Focal Laser Ablations since 2011.
Recent publications from Dr. Walser and his team
Focal Laser Ablation of prostate cancer: results in 121 patients with low to intermediate risk disease
Eric Walser, Anne Nance, Leslie Ynalvez, Shan Yong, Jacqueline Aoughsten, Eduardo Eyzaguirre, Stephen Williams UTMB, Galveston TX, USA
Background: Focal laser ablation (FLA) is an alternative to whole-gland therapies for localized prostate cancer
Objective: Determine the morbidity and oncologic and functional outcomes at one year in the largest FLA series to date.
Design, Setting and Participants: 121 consecutive patients with low to intermediate risk prostate cancer had FLA between 2013 and 2016. At 6 and 12 months, patients had clinical follow up and prostate MRI with biopsies of suspicious areas. Surveys of sexual and urinary function before and after FLA documented functional outcomes.
Intervention: Transrectal, MRI-guided FLA done with a laser fiber inserted into affected portions of the prostate. MRI thermometry-controlled ablation location/size.
Statistical Analysis: Multivariate logistic regression analyses identified determinants of positive post- treatment biopsy. 2-sided Wilcoxon signed rank test for survey scores and lab values.
Results and Limitations: The median age was 64 and median PSA was 6.05 ng/mL. Gleason score was 6 in 36 (29.8%) and 7 in 85 (70.2%), respectively. Fourteen (11.6%) of men had clinically-significant cancer after biopsy of MRI-abnormal areas after FLA. There was no significant difference between baseline and functional scores post-ablation. As the hemigland ablation technique emerged, there were improved results and only one of 16 (6.8%) men had residual cancer at one year. The median PSA fell to 3.25 at 12 months (p<0.001). Other than lesion size, there were no predictors for positive post- treatment prostate biopsy.
Conclusions: At 1 year, FLA in selected patients has promising oncologic results, low morbidity and no significant changes in quality of life.
Patient Summary: This study looks at the largest series to date of 121 men with prostate cancer treated with focal laser ablation --a non-surgical, outpatient procedure for low to intermediate risk prostate cancer. At one year, only 12% of men (14 of 121) had biopsy-proven significant prostate cancer. Sexual and urinary function did not change after FLA.
https://www.focaltherapy.org/2018/ABSTRACTS/FT2018_PP49.pdf
Dr. Sangeet Ghai is the Director of the Biopsy Centre at JDMI, a member of the Princess Margaret Cancer Clinical Research Unit, and an Associate Professor in the Department of Medical Imaging at the University of Toronto. He completed his postgraduate training in India and did a dual fellowship in abdominal imaging and interventional radiology at the University of Toronto.
Dr. Ghai is responsible for the clinical study with CLS products in Toronto, the study is co-sponsored by CLS and the University Health Network in Toronto.
“This clinical research study is designed to determine the ability of in bore MRI guided Focal Laser Ablation (MRgFLA) in patients with early stage carcinoma of prostate. The results will be evaluated by repeated MRI and prostate biopsy. Previous prospective development study demonstrated that FLA may be a viable option for men with low-intermediate risk prostate cancer. The vast majority of patients undergoing this treatment experienced minimal side effects with no peri-operative complications. Over 80% of patients treated with MRgFLA remain on AS and were able to avoid radical therapy at mean follow up duration of 3 years.”
“Study Objectives are to evaluate the proportion of patients with low-intermediate risk prostate cancer undergoing focal laser ablation (MRgFLA) prostate treatment who will be free of clinically significant PCa, when using a > 5mm margins around the MR visible tumor in defining the ablation contour. The rationale of the study is to show that MRgFLA is a safe procedure that can significantly postpone or eliminate the need for patients with Low-Intermediate Risk prostate cancer to undergo a definitive treatment (i.e., Radical Prostatectomy or Radiation therapy) for their disease. Study population: patients with Low-Intermediate Risk Prostate Cancer who are willing to take part in the study and meet study eligibility criteria. Primary Study Objective is to show that MRgFLA can significantly reduce the need for definitive treatment (e.g., Radical Prostatectomy, or Radiation therapy) and is a safe procedure for patients with Low-Intermediate Risk Prostate Cancer defined in the current protocol as 1) Gleason score 6 and 7 (=3+4 or 4+3; No Grade 5 pattern), and 2) T1-T2, N0, M0.”
ClinicalTrials.gov Identifier: NCT03650595
Dr. John F. Feller, founding partner of Desert Medical Imaging, currently the owner and Medical Director of the practice.
Board-certified as a Diagnostic Radiologist with sub-specialties in Orthopedic & Sports Medicine Imaging, Body MRI, and holds a Level II Cardiac CT certification. Dr. Feller is also a partner in China’s first American-owned multi-disciplinary outpatient healthcare facility and serves as director of radiology.
Recent publications from DMI – displaying 8-year data
PURPOSE: In the United States alone, new prostate cancer cases for 2017 were estimated at 161,360 and deaths at 26,730 according to the AACR. Focal therapies for low risk and intermediate risk localized prostate cancer are increasingly being explored.
METHODS AND MATERIALS: All MRI-guided therapy was delivered using a 1.5 Tesla Philips AchievaXR system (Philips Healthcare, Best, The Netherlands) for both image acquisition and real-time thermometry. DynaCADand DynaLOC(Invivo, Orlando, FL, USA) software were used for image analysis and interventional planning using the DynaTRIMpositioning hardware (Fig. a) (Invivo, Orlando, FL, USA). Laser therapy was delivered using a Visualase(Medtronic, Minneapolis, MN, USA) 15W, 980 nm diode laser (Fig. b) with cooled (Medtronic, Minneapolis, MN, USA) or non-cooled (Clinical Laserthermia Systems, Lund, Sweden) laser fiber introduced transrectally.
RESULTS: Mean PSA dropped 38%, 12 mos. post-treatment. 95% CI shown as error bars (Table 1). We observed more pronounced PSA decline (~50%) in the salvage cohort and attributed this to their comparatively high PSAs relative to the treatment naïve cohort. Compared to the initial PSA (Month 0), paired Student’s t-test used to evaluate mean PSA, p<.001***. No patient experienced permanent erectile dysfunction or incontinence as a result of treatment. While no prostate cancer-specific deaths have occurred, a Kaplan-Meier Curve of recurrent cancer is shown with 95% confidence interval bands (Table 2). The drop at the 6-month mark is due to the research protocol which requires that an MRI-guided biopsy be acquired from the treatment site (even in the absence of 6month following treatment to detect marginal recurrence). Kaplan-meier curves in Tables 3 and 4 illustrate the low rate of metastasis and death in both the treatment naïve and salvage cohorts; however, two patients expired from metastatic melanoma.
CONCLUSIONS: Eight-year interim results in over 100 patients indicates that outpatient, MRI-guided, transrectal laser focal therapy is both safe and feasible. No statistically significant erectile dysfunction or incontinence occurred. Short-term and intermediate-term oncologic control is achievable in 75% of patients that include both treatment naïve and salvage inclusion criteria.
Full poster at: http://assets.auanet.org/SITES/AUAnet/PDFs/AUA2018-Posters-MP30-02.pdf
Forthcoming
Upon reading a document presented by DMI´s Bernadette Greenwood at Brigham and Womens Hospital back in May this year, some very interesting information was found regarding the next steps for FLA. First, an international multi-institutional phase 2 trial through the International Laser Network is awaiting IRB approval. Second, an IND submission has been completed to FDA for combination therapy and its awaiting approval.


ILN Participants
· Desert Medical Imaging, Indian Wells, CA
· MD Anderson Cancer Center Houston, TX
· Radboud University, Nijmegen, The Netherlands
· The Mayo Clinic, Rochester, MN
· National Cancer Institute, Bethesda, MD
· University of California, Los Angeles, CA
· University of Chicago, Chicago, IL
· University of Toronto, Ontario, Canada
· University of Texas Medical Branch, Galveston, TX
· Laser Prostate Centers of America, Houston, TX
· Weill Cornell, New York, NT
· Prostate Laser Center, Houston TX
The fact that the above members are coordinating a multi-institutional study is indeed exciting and to quote a headline from an article in Urology Times published in Oct this year,
“Focal therapy for prostate Cancer: Ready for prime time?”
Well, it sure looks like it when these cutting-edge opinion leading hospitals coordinate resources to bring Focal Laser Ablation into the standard of care and with continued good results reimbursement will follow. And regarding FLA as a standard of care, it has already begun, the University of Chicago according to an interview with Dr. Oto Aytekin in AJR, they are already offering FLA as their standard of care.
“At the Sanford J. Grossman Center of Excellence in Prostate Imaging and Image Guided Therapy at the University of Chicago, we are the pioneers in MR guided Focal Laser Ablation and successfully completed Phase I and NIH funded Phase II trials and now offer this procedure as standard of care at our institution.”
Even though some pieces are yet to be laid in the puzzle of disrupting a long-time grid locked treatment rationale which emphasis cutting away half or the entire prostate while gambling with the patients quality of life, things are moving rapidly for the minimally invasive FLA method. The technological advancements in imaging and guidance along with cutting edge non-cooled diffuser fiber technology provided by CLS puts the laser in line to be a serious contender to the scalpel in several ways.
CLS goals for 2018 are quickly being met as the company now as signed several agreements aiming to enhance its market offering which in turn should speed up their commercialization. Through the global licensing agreement with the key market player MRI Interventions Inc. CLS now enters into vast new market segments and the once single legged business model now has multiple indications and addresses not only treatment of solid tumors and hyperplasia but also non-malignant vascular malformations. The neurosurgical segment addresses diseases like Epilepsy and Parkinson’s which alone represent huge market opportunities.
The company´s unique imILT method is as stated above heading for a milestone and the next steps include a larger clinical study aiming to exploit it´s full immunological potential. And the questions awaiting an answer is, will the imILT method be accompanied by a immunostimulant drug? And will leading players in the field of checkpoint inhibitors, like Merck or Bristol Myers Squibb, join CLS as partners in this combinatorial treatment quest?
The thing that makes you scratch your head and another question lacking an answer is, when will the giant MedTech conglomerates like Medtronic act? Are they really neglecting the fact that well renowned hospitals and clinicians are turning their eyes and money to CLS technology and in some cases using CLS diffuserfiber together with the Visualase system, or, are they in some way already involved? The future holds the answer and perhaps the answer doesn’t lie with them but instead with the undisclosed leading hospital that signed a supplier agreement with CLS and has made repeat orders which serve as proof of concept for their treatment of symptomatic non-malignant vascular malformations. For good reasons I still believe this leading hospital is none lesser than the world-renowned Mayo Clinic.
The forthcoming follow-up results from CLS clinical study’s together with further business activities will be the primary short-term drivers for the stock and in regard to clinical results, the median overall survival for the imILT treated pancreatic cancer patients are heading for somewhat revolutionary figures, 17 months and counting.